Guest Column: Keeping Up with the Crustaceans
This article was written by Dr. Danai Georgiadou, a scientist from A*STAR’s Bioprocessing Technology Institute (BTI). She also contributes to CRISP Meats, a multi-institutional research programme led by A*STAR to address challenges faced by the industry, and accelerate the development and production of cultivated meat and seafood through public-private partnerships.
Cellular agriculture is a dynamic field that is emerging as a vital part of building a secure and sustainable food future in Asia. Since the first public debut of cultivated beef in 2013, remarkable progress has been made in the cultivated meat industry. However, the development of cultivated seafood from crustaceans such as shrimp, lobsters, and crabs has lagged behind, contributing to a growing sense of concern about the significant challenges associated with this process.
This deficit is not due to a lack of R&D attempts—there have been more than 500 studies published since the 1960s on the topic of crustacean cell cultivation. Rather, it is due to the complications of working with a diverse species range and a persistent knowledge gap in their various metabolic requirements. The development of stable crustacean cell lines is crucial not only for the advancement of alternative proteins but also as a model for studying viral infections that have occurred primarily due to intensive farming over the past two decades, resulting in severe consequences for aquatic ecosystems.

Why are crustacean cell lines more challenging to cultivate and immortalise than mammalian cell lines?
When planning to establish a novel cell line from a species, it is crucial to consider the animal’s natural environment, physiology, and metabolism. This understanding is necessary to recapitulate the intra- and extracellular conditions of different cell types as closely as possible in vitro, such that the cells maintain their natural characteristics and behaviour. However, the culture conditions that have reportedly been utilised to establish crustacean cell lines often do not adhere to this principle. This is due to significant differences between aquatic and atmospheric environments, and the challenges of precisely replicating relevant physiological conditions outside the body of the animal.
Oxygen and pressure
Many of us have likely experienced discomfort or pain in our ears and sinuses when diving into the bottom of a swimming pool. This sensation is caused by the increased pressure in the water, which compresses the cavities in our bodies that contain air. As we venture deeper into the ocean, the pressure only intensifies. This raises the question of how crustaceans and other aquatic organisms can survive in deep parts of the ocean.
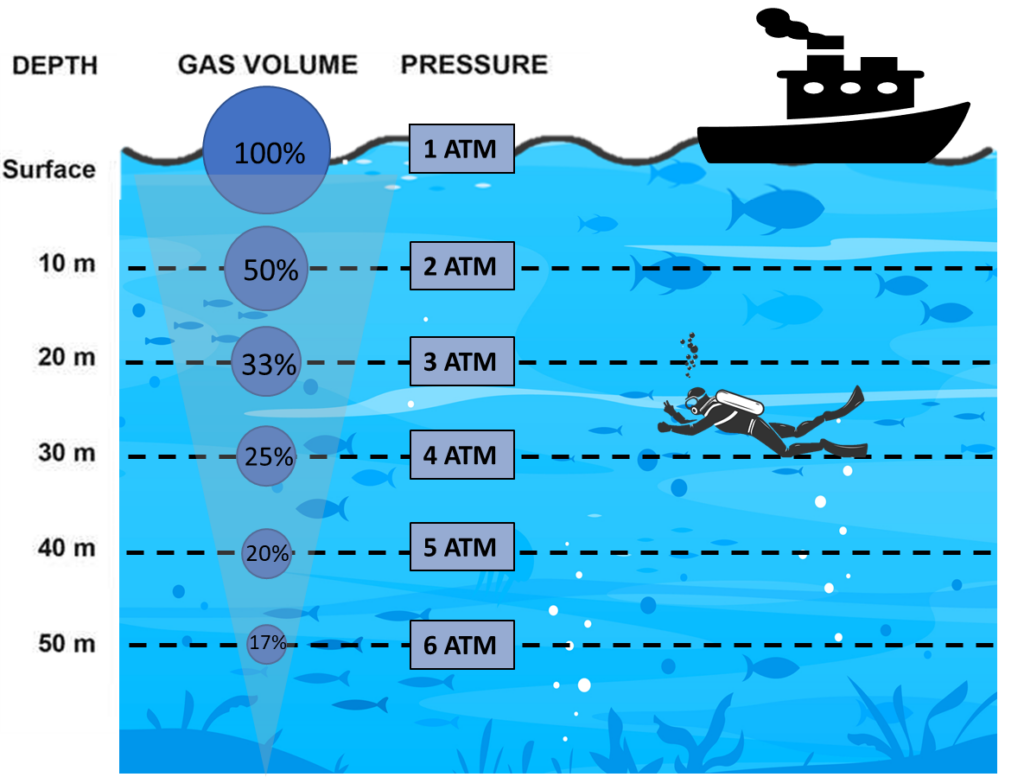
Unlike mammals, who rely on air-filled cavities within their body for survival, aquatic animals have adapted to their environment by eliminating these pockets of air. This isn’t the only adaptation that allows aquatic animals to cope with life in a high-pressure environment. High pressure also increases the speed of chemical reactions in the cells, which drives the metabolism of these organisms to deviate significantly from those of organisms that live on the surface. A recent study revealed the existence of additional biomolecules that protect organisms from the damaging effects of high pressure—a finding that relates to the unique metabolism of crustaceans and other non-mammalian aquatic animals.
The same principle applies to the adaptation of organisms in a low-oxygen or hypoxic environment. Again, chemical reactions are altered and metabolism is adjusted. Even in mammalian cell culture, hypoxia has been shown to give a growth advantage to the cells. This comes as no surprise since oxygen concentrations within our bodily tissues are significantly lower than the 20-21 percent oxygen present in the atmosphere. The abiotic factors of pressure and oxygen have seldom posed a challenge for culturing cells of mammalian species that coexist with us under atmospheric conditions. However, cultivating the cells of organisms such as lobsters and shrimp, which can be found in habitats more than 500 metres deep, necessitates additional research into their metabolic adaptations and the challenges presented when these animals and their isolated cells are brought to the surface for study.
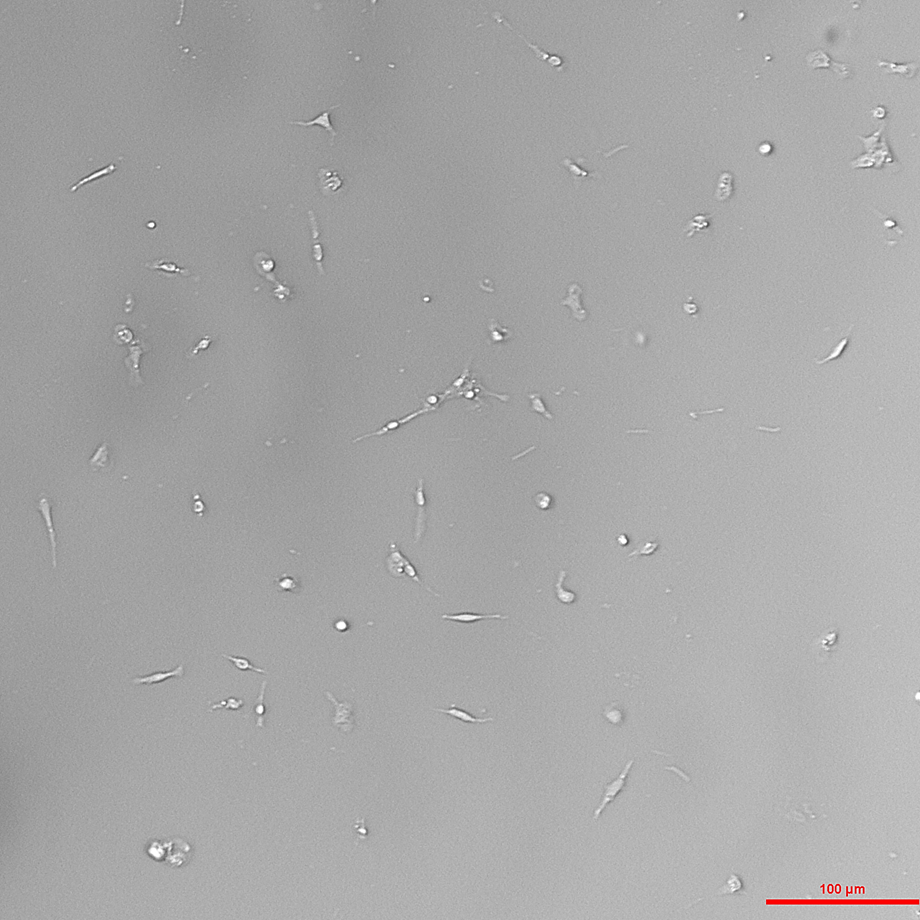
A microscopy image of shrimp cells in culture
(Courtesy of Dr. Danai Georgiadou)
Osmolality
Osmolality refers to the concentration of substances dissolved in a liquid and is a measure of how concentrated or salty the liquid is. It affects the movement of water and substances across cell membranes and plays a role in maintaining cellular balance and health. Crustacean cells, like those of most animals, thrive in a unique osmotic environment that is required for normal functioning.
To retain optimal cell properties and performance in vitro, the osmolality of a culture medium should closely match that of a cell’s natural habitat. Correcting osmolality in a culture medium is a relatively simple task, but ignoring it can be detrimental to the cells. Failure to maintain the appropriate osmotic balance can lead to cellular stress, osmotic shock, and even cell death. Therefore, it is essential to consider the osmolality of the natural habitat of the cells we are working with and accordingly adjust every solution and buffer used for their culture.
Extracellular matrix
In cell biology, the extracellular matrix (ECM) refers to the complex mixture of proteins, carbohydrates, and other macromolecules that surround and support cells in their natural environment. The ECM plays a crucial role in regulating cell behaviour, including cell adhesion, migration, proliferation, and differentiation. It provides structural support, biochemical cues, and mechanical signals that influence cellular functions. Researchers have developed methods to incorporate ECM components into cell culture systems to better mimic the native tissue microenvironment. Crustaceans appear to have a distinct composition of ECM that differs significantly from those of mammals. The primary ECM component found in crustaceans is chitin—a complex polysaccharide that serves as the primary structural component of crustacean exoskeletons. As such, it stands to reason that commonly used ECM proteins in human and mouse cell line research—such as collagen and gelatin—may not be effective for promoting crustacean cell growth in vitro. To create a more suitable cell culture environment, it is necessary to develop matrices that closely resemble the structural and molecular features found in crustacean tissues in vivo.
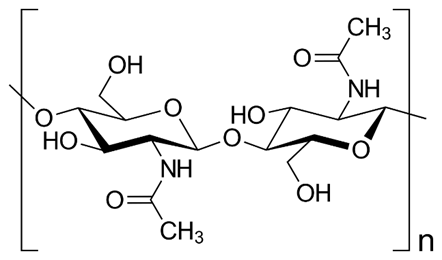
The chemical structure of chitin
How can we effectively address these challenges?
To overcome the hurdles facing the development of crustacean cell lines, a smarter and more comprehensive research approach is needed. Before we can develop a cell line for cultivated meat purposes, many fundamental scientific questions must be answered. Increased collaboration between research institutes or universities and cultivated seafood companies would intensify research efforts that focus on understanding the fundamental biology of distinct crustacean cell types and designing optimal culture conditions to support them. Such foundational research will also aid in the development of much-needed research tools, such as validated antibodies and annotated genome sequences, which are severely lacking for aquatic species.
A more coordinated approach is also necessary, with researchers sharing their results and working together to fill knowledge gaps in the metabolic requirements of these organisms. By doing so, we can unlock the true potential of aquatic invertebrates and pave the way for new discoveries that can propel the cultivated seafood field.
If you are interested in collaborating with BTI and CRISP Meats on crustacean cell culture, please reach out to Dr. Danai Georgiadou at Danai_Georgiadou@bti.a-star.edu.sg.
